Abstract
Review Article
Exploring pathophysiology of COVID-19 infection: Faux espoir and dormant therapeutic options
Vinod Nikhra*
Published: 05 May, 2020 | Volume 4 - Issue 1 | Pages: 034-040
COVID-19 virus structural components: The 2019-nCoV, also called SARS-CoV-2, was first reported in Wuhan, China in December 2019. The disease was named Coronavirus Disease 2019 (COVID-19) and the virus responsible for it as the COVID-19 virus, respectively, by WHO. The 2019-nCoV has a round, elliptic or pleomorphic form with a diameter of 60–140 nm. It has single-stranded RNA genome containing 29891 nucleotides, a lipid shell, and spike, envelope, membrane and hemagglutinin-esterase (HE) proteins.
Steps in progression of COVID-19 illness: Once inside the airways, the S protein on the viral surface recognizes and mediates the attachment to host ACE-2 receptors and gains access to endoplasmic reticulum. The HE protein facilitates the S protein-mediated cell entry and virus spread through the mucosa, helping the virus to attack the ACE2-bearing cells lining the airways and infecting upper as well as lower respiratory tracts. With the dying cells sloughing down and filling the airways, the virus is carried deeper into the lungs. In addition, the virus is able to infect ACE2-bearing cells in other organs, including the blood vessels, gut and kidneys. With the viral infestation, the activated immune system leads to inflammation, pyrexia and pulmonary edema. The hyperactivated immune response, called cytokine storm in extreme cases, can damage various organs apart from lungs and increases susceptibility to infectious bacteria especially in those suffering from chronic diseases.
The current therapeutics for COVID-19: At present, there is no specific antiviral treatment available for the disease. The milder cases may need no treatment. In moderate to severe cases, the clinical management includes infection prevention and control measures, and symptomatic and supportive care, including supplementary oxygen therapy. In the critically ill patients, mechanical ventilation is required for respiratory failure and hemodynamic support is imperative for managing circulatory failure and septic shock.
Conclusion: Confusion, despair and hopes: There is no vaccine for preexposure prophylaxis or postexposure management. There are no specific approved drugs for the treatment for the disease. A number of drugs approved for other conditions as well as several investigational drugs are being canned and studied in several clinical trials for their likely role in COVID-19 prophylaxis or treatment. The future seems afflicted with dormant therapeutic options as well as faux Espoir or false hopes. As obvious, not all clinical trials will be successful, but having so many efforts in progress, some may succeed and provide a positive solution. Right now, though, confusion and despair prevail.
Read Full Article HTML DOI: 10.29328/journal.ijcv.1001013 Cite this Article Read Full Article PDF
Keywords:
COVID-19; 2019 nCoV; Spike (S) protein; ACE-2; Cytokine storm syndrome; Azithromycin; Chloroquine; Hydroxychloroquine; Remdesivir; COVID-19 vaccine
References
- Naming the coronavirus disease (COVID-19) and the virus that causes it. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
- Lu R, Zhao X, Li J, Niu P, Yang B, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020; 395: 565-574. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32007145
- Chan JF, Kok KH, Zhu Z, Chu H, To KK, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020; 9: 221-236. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31987001
- Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015; 1282: 1–23. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25720466
- Li Q, Guan X, Wu P, Wang X, Zhou L, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020; 382: 1199-1207. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31995857
- Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. et al. Features, Evaluation and Treatment Coronavirus (COVID-19). StatPearls Publishing LLC. Bookshelf ID: NBK554776. 2020; PMID: 32150360. PubMed: https://www.ncbi.nlm.nih.gov/books/NBK554776/
- Porter DL, Maloney DG. Cytokine release syndrome (CRS). UpToDate. 2020. Retrieved from https://www.uptodate.com/contents/cytokine-release-syndrome-crs?
- Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Resp Med. Corres. 2020. https://www.ncbi.nlm.nih.gov/pubmed/32353251 PubMed:
- Angeletti S, Benvenuto D, Bianchi M, Giovanetti M, Pascarella S, et al. COVID-2019: The role of the nsp2 and nsp3 in its pathogenesis. J Med Virol. 2020.
- Lei J, Kusov Y, Hilgenfeld R. Nsp3 of coronaviruses: Structures and functions of a large multi-domain protein. Antiviral Res. 2018; 149: 58-74. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29128390
- Tai W, He L, Zhang X, Pu J, Voronin D, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020. https://www.ncbi.nlm.nih.gov/pubmed/32203189
- Xu X, Chen P, Wang J, Feng J, Zhou H, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modelling of its spike protein for risk of human transmission. Sci China Life Sci. 2020; 63: 457-460. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32009228
- Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020; 92: 418-423. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31967327
- Kuba K, Imai Y, Rao S, Gao H, Guo F, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2020; 11: 875-879. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16007097
- Xu H, Zhong L, Deng J, Peng J, Dan H, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020; 12: 8.
- In the author’s opinion – inferring on the available findings and research data.
- Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2019. https://www.nejm.org/doi/full/10.1056/NEJMoa2001017
- Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y,et al. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. 2020.
- Cao Y, Li L, Feng Z, Wan S, Huang P, et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020; 6: 11. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32133153
- Guan WJ, Ni Z, Hu Y, Liang W, Ou C, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N England J of Med. www.medrxiv.org/content/10.1101/2020.02.06.20020974v1
- Huang C, Wang Y, Li X, Ren 4, Zhao J, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395: 497-506. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31986264
- In the author’s opinion – inferring on the available findings and research data.
- Anosmia, hyposmia and dysgeusia in the absence of other relevant disease should alert to the possibility of COVID-19 infection and warrant consideration for testing. American Academy of Otolaryngology-Head and Neck Surgery – position statement.
- Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020; 51: 843-851. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32222988
- Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32091533
- Lianhan S, Jianping Z, Yi H, Du Ronghui, Bin C. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020.
- Baden LR, Rubin EJ. Covid-19 - The Search for Effective Therapy. 2020. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32187463
- Cao B, Wang Y, Wen D, Liu W, Wang J, et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32187464
- Huang J. Efficacy of Chloroquine and Lopinavir/ Ritonavir in mild/general novel coronavirus (CoVID-19) infections: a prospective, open-label, multicenter randomized controlled clinical study. 2020. http://www.chictr.org.cn/showproj.aspx?proj=49263
- https://www.the-scientist.com/news-opinion/flu-and-anti-hiv-drugs-show-efficacy-against-coronavirus-67052
- Chen C, Zhang Y, Huang J, Yin P, Cheng Z, et al. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. 2020. www.medrxiv.org/content/10.1101/2020.03.17.20037432v1
- precisionvaccinations.com/avigan-favipiravir-t-705-broad-spectrum-inhibitor-viral-rna-polymerase
- Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020; 295: 4773-4779. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32094225
- Wang M, Cao R, Zhang L, Yang X, Liu J, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020; 30: 269-271. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32020029
- Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020; 105949. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32205204
- Liu J, Cao R, Xu M, Wang X, Zhang H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020; 6: 16. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32194981
- Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D, et al. Chloroquine and hydroxychloroquine as available weapons to Figureht COVID-19. Int J Antimicrob Agents. 2020; 105932. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32145363
- Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005; 2: 69. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16115318
- Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020; 14: 72-73. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32074550
- Yao X, Ye F, Zhang M, Cui C, Huang B, et al. in vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020; pii: ciaa237. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32150618
- Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. in vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004; 323: 264-268. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15351731
- Yao X, Ye F, Zhang M, Cui C, Huang B, et al. in vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020; pii: ciaa237. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32150618
- Information on registered clinical trials for COVID-19 in the United States is available at: https://clinicaltrials.gov/external icon
- Duan K, Liu B, Li C, Zhang H, Yu T, et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. 2020.
- https://www.boston.com/news/health/2020/03/24/when-might-experimental-drugs-to-treat-covid-19-be-ready
- Zhou D, Dai SM, Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrobial Chemotherapy. 2020; dkaa114.
- cnbc.com/2020/03/17/hopes-of-a-coronavirus-vaccine-mount-as-three-key-biotech-players-make-progress.html
Figures:
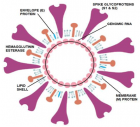
Figure 1
Figure 2

Figure 3
Similar Articles
-
Pseudoephedrine protects mice from infection of H1N1 virusZhongping Wu*,Li Deng,Chengzhi Chu,Xiaoyin Chen*. Pseudoephedrine protects mice from infection of H1N1 virus. . 2020 doi: 10.29328/journal.ijcv.1001008; 4: 014-020
-
The Psychology of the Common Cold and Influenza: Implications for COVID-19Andrew P Smith*. The Psychology of the Common Cold and Influenza: Implications for COVID-19. . 2020 doi: 10.29328/journal.ijcv.1001011; 4: 027-031
-
Yemen is free of COVID-19Hussein O Kadi*. Yemen is free of COVID-19. . 2020 doi: 10.29328/journal.ijcv.1001012; 4: 032-033
-
Exploring pathophysiology of COVID-19 infection: Faux espoir and dormant therapeutic optionsVinod Nikhra*. Exploring pathophysiology of COVID-19 infection: Faux espoir and dormant therapeutic options. . 2020 doi: 10.29328/journal.ijcv.1001013; 4: 034-040
-
COVID-19: Targeting the cytokine storm via cholinergic anti-inflammatory (Pyridostigmine)Ahmed H Osman*. COVID-19: Targeting the cytokine storm via cholinergic anti-inflammatory (Pyridostigmine). . 2020 doi: 10.29328/journal.ijcv.1001014; 4: 041-046
-
Identifying patterns in COVID-19: Morbidity, recovery and the aftermathVinod Nikhra*. Identifying patterns in COVID-19: Morbidity, recovery and the aftermath. . 2020 doi: 10.29328/journal.ijcv.1001016; 4: 056-064
-
Role of nanotechnology in diagnosing and treating COVID-19 during the PandemicAbdul Baset*,Abdul Waris,Muhammad Ali,Atta Ullah Khan,Asmat Ali. Role of nanotechnology in diagnosing and treating COVID-19 during the Pandemic. . 2020 doi: 10.29328/journal.ijcv.1001017; 4: 065-070
-
COVID-19: The possible medical strategiesMohamed SA Mohamed*. COVID-19: The possible medical strategies. . 2020 doi: 10.29328/journal.ijcv.1001018; 4: 071-075
-
Inhaled statins to combat COVID-19 – prophylactic and treatment approachArchana P Iyer*,Maryam A Al-Ghamdi. Inhaled statins to combat COVID-19 – prophylactic and treatment approach. . 2020 doi: 10.29328/journal.ijcv.1001020; 4: 079-080
-
A Comprehensive review on genomic diversity and epidemiology of COVID-19Zeshan Haider Raza*,Muhammad Ahmed Ihsan,Sahrish Khan,Haroon Zafar,Tayyaba Rehman. A Comprehensive review on genomic diversity and epidemiology of COVID-19. . 2020 doi: 10.29328/journal.ijcv.1001021; 4: 081-095
Recently Viewed
-
Do Fishes Hallucinate Human Folks?Dinesh R*,Sherry Abraham,Kathiresan K,Susitharan V,Jeyapavithran C,Paul Nathaniel T,Siva Ganesh P. Do Fishes Hallucinate Human Folks?. Arch Food Nutr Sci. 2017: doi: 10.29328/journal.afns.1001003; 1: 020-023
-
Assessment of Redox Patterns at the Transcriptional and Systemic Levels in Newly Diagnosed Acute LeukemiaAna Carolina Agüero Aguilera, María Eugenia Mónaco, Sandra Lazarte, Emilse Ledesma Achem, Natalia Sofía Álvarez Asensio, Magdalena María Terán, Blanca Alicia Issé, Marcela Medina, Cecilia Haro*. Assessment of Redox Patterns at the Transcriptional and Systemic Levels in Newly Diagnosed Acute Leukemia. J Hematol Clin Res. 2024: doi: 10.29328/journal.jhcr.1001029; 8: 017-023
-
Assessment of Indigenous Knowledge on Using of Traditional Medicinal Plants to Cure Human Diseases in South Omo Zone Baka Dawla Ari District, Kure and Bitsmal South EthiopiaGizaw Bejigo*. Assessment of Indigenous Knowledge on Using of Traditional Medicinal Plants to Cure Human Diseases in South Omo Zone Baka Dawla Ari District, Kure and Bitsmal South Ethiopia. J Plant Sci Phytopathol. 2024: doi: 10.29328/journal.jpsp.1001132; 8: 048-054
-
Nanoencapsulated Extracts from Leaves of Bauhinia forficata Link: In vitro Antioxidant, Toxicogenetic, and Hypoglycemic Activity Effects in Streptozotocin-induced Diabetic MiceBárbara Verônica Cardoso de Souza, Alessandra Braga Ribeiro*, Rita de Cássia Meneses Oliveira, Julianne Viana Freire Portela, Ana Amélia de Carvalho Melo Cavalcante, Esmeralda Maria Lustosa Barros, Luís Felipe Lima Matos, Tarsia Giabardo Alves, Maria. Nanoencapsulated Extracts from Leaves of Bauhinia forficata Link: In vitro Antioxidant, Toxicogenetic, and Hypoglycemic Activity Effects in Streptozotocin-induced Diabetic Mice. Arch Pharm Pharma Sci. 2024: doi: 10.29328/journal.apps.1001063; 8: 100-115
-
GELS as Pharmaceutical Form in Hospital Galenic Practice: Chemico-physical and Pharmaceutical AspectsLuisetto M*,Edbey Kaled,Mashori GR,Ferraiuolo A,Fiazza C,Cabianca L,Latyschev OY. GELS as Pharmaceutical Form in Hospital Galenic Practice: Chemico-physical and Pharmaceutical Aspects. Arch Surg Clin Res. 2025: doi: 10.29328/journal.ascr.1001084; 9: 001-007
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."