More Information
Submitted: October 13, 2022 | Approved: November 14, 2022 | Published: November 15, 2022
How to cite this article: Srinivasa S. Comparative study of once daily tacrolimus (extended-release capsule) versus conventional twice daily tacrolimus in renal transplant recipients. Int J Clin Virol. 2022; 6: 050-054.
DOI: 10.29328/journal.ijcv.1001050
Copyright License: © 2022 Srinivasa S. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: OD Tacrolimus; Post-renal transplant; Immunosuppressant
Comparative study of once daily tacrolimus (extended-release capsule) versus conventional twice daily tacrolimus in renal transplant recipients
Sanjay Srinivasa*
Chief Nephrologist & Transplant Physician, Department of Nephrology, Suguna Hospital, Bangalore, India
*Address for Correspondence: Dr. Sanjay Srinivasa, Chief Nephrologist & Transplant Physician, Department of Nephrology, Suguna Hospital, Bangalore, India, Email: [email protected]; [email protected]
Background: One of the common causes of chronic allograft nephropathy is nonadherence to medications, contributing to 30% of graft loss in the developed world. The non-adherence is attributed predominantly to pill burden.
Once-daily dosing of tacrolimus instead of conventional twice-daily dosing may enhance adherence to medication and improve long-term outcomes. The present study is a retrospective analysis comparing the safety and effectiveness of De Novo (use from day 1) once daily (OD) Tacrolimus (extended-release capsules) to conventional twice-a-day (BD) tacrolimus, in renal transplant recipients operated at Suguna Hospital Bangalore India.
Material and Methods: Records of 24 Transplant patients on De novo OD Tacrolimus were analyzed and compared retrospectively to 24 transplant patients treated De Novo with conventional BD tacrolimus on a regular follow-up for at least 2 yrs. post-transplant at our center.
Results: Various parameters recorded till the last follow-up were analyzed and compared. The average weight of the cohort (64.6 kg vs. 66.6 kg), average tacrolimus dose (2.7 mg vs. 2.15 mg), average Tac dose/kg body weight (0.04 mg vs. 0.03 mg), average Sr.Creatinine, at Last, Follow up (1.2 mg/dl vs. 1.32 mg/dl) were comparable in both groups and were statistically insignificant (p > 0.05). However, there was a higher incidence of Post-Transplant Diabetes Mellitus (PTDM) noted in the Conventional BD Tacrolimus group (20.83%) compared to the OD Tacrolimus group (4.1%) and it was statistically significant (p = 0.17). The Infection rate of 41.67% in the conventional BD Tacrolimus group was much higher compared to the OD Tacrolimus group (4.17%) which was statistically significant (p = 0.01). There was 100% patient and graft survival at the end of two years in both groups. Tacrolimus Dose for OD and Conventional BD dosing were similar, unlike earlier studies recommending a 10% increase in dose for OD tacrolimus compared to BD dosing.
Conclusion: OD Tacrolimus dose is comparable to conventional BD dose Tacrolimus in its safety and Efficacy; however, it scores over conventional BD dose Tacrolimus in terms of post-transplant infections and post-transplant diabetes mellitus (PTDM) and a more stable serum trough level.
Tacrolimus, a calcineurin inhibitor, is derived from the soil fungus Streptomyces tsukubaensi, found in Japan [1]. Tacrolimus has presented a notable decrease in the frequency and severity of acute allograft rejection episodes in solid organ (kidney, liver, and heart) transplants with enhanced long-term graft survival [2].
Tacrolimus use is associated with a number of adverse effects like nephrotoxicity, neurotoxicity, new-onset diabetes, hyperkalemia, hypertension, hyperlipidemia, hypomagne-semia, and hyperuricemia [3]. A new formulation of tacrolimus i.e., tacrolimus extended-release can be dosed once daily (OD) [4] and may have the ability to simplify immunosuppressive regimens and improve medication compliance translating to better long-term allograft survival [5]. OD Tac (tacrolimus extended-release capsules) is indicated for prophylaxis of organ rejection in adult patients receiving allogeneic kidney and liver transplants.
Once-daily dosing of tacrolimus instead of twice-daily dosing may enhance adherence to medication and improve long-term outcomes [6]. The present study is a retrospective analysis comparing the safety and efficacy of de novo (use from day 1) OD Tac (tacrolimus extended-release capsules) to de novo conventional twice-a-day tacrolimus, among renal transplant recipients transplanted at Suguna hospital, with at least 2 yrs. post-transplant follow-up.
This is a single-center retrospective analysis of data obtained from 24 consecutive patients started on de novo OD tacrolimus vs. 24 consecutive patients on de novo Conventional BD Tacrolimus followed up for at least 2 yrs. post-transplant, in the department of nephrology, at Suguna Hospital, Rajajinagar, Bangalore, India. The study was approved by the institutional ethical committee. Data were obtained from case records of patients who underwent kidney transplantation and were on regular follow-ups at our center for at least 2 yrs. Patients, less than 18 years of age, on irregular follow-up, Patients with previous renal or non-renal transplants, and switch-over patients to OD Tac from conventional Tac were excluded from the study.
All patients were on a Standard immunosuppressive regimen consisting of Tacrolimus, Mycophenolate Mofetil, and Steroids. Induction therapy was given with two doses of Basiliximab. Our transplant protocol includes the use of Induction in more than 3 mismatches, emotionally related and cadaveric transplants. Tacrolimus is initiated on day 5 at a dose of 0.05 mg/kg twice daily in patients on BD tacrolimus and 0.1 mg/kg as a single dose in patients on OD tacrolimus, to obtain Tac level on day 0 of transplant. Tacrolimus 5th day trough level is obtained on the day of the transplant, which helps adjust the dose to avoid vast variations in levels in the immediate post-transplant period. Tacrolimus dose is adjusted to achieve a target T0 level of 5 ng/ml - 9 ng/ml during immediate post-transplant and up to the first 3 months post-transplant. The dose is adjusted to target levels of 3 ng/ml to 5 ng/ml beyond 3 months post-transplant. MMF is initiated on day 1 at a dose of 0.5 g twice daily. Prednisolone is initiated at a dose of 30mg the previous night of transplant, IV Methylprednisolone 1 g is used at the time of clamp release, followed by, 500 mg and 250 mg on days 1 and 2 post-transplant respectively. Prednisolone is started at 20 mg OD on POD3 and is tapered 2.5 mg every 15 days to achieve a dose of 7.5 mg OD daily by 10 weeks.
Patient data were divided into two groups, patients receiving De novo OD Tac (tacrolimus extended-release capsules, one/day) and those on conventional tacrolimus (two times/day).
The primary objective of the study was to compare the data of the 2 groups with respect to initial Tacrolimus Dose and T0 Trough levels on Day 0 transplant, Creatinine at discharge, Creatinine at last visit, and average Tac dose/kg body weight. Between these 2 groups. Data were also collected to compare side effect profiles, the incidence of post-transplant diabetes mellitus (PTDM), infections, and rejection episodes.
Statistics
Collected data was compiled using Microsoft Excel, and analyzed using SPSS 23.0 version. Frequency, percentage, means, and standard deviations (SD) were calculated for the continuous variables, while ratios and proportions were calculated for the categorical variables. The difference of proportions between qualitative variables was tested using the chi-square test or Fisher exact test as applicable. A p - value less than 0.5 was considered statistically significant.
In the present study, general parameters such as age, gender, body weight, type of donor, and time since treatment were comparable among both groups, and the difference was not statistically significant (p > .05) Table 1.
| Table 1: Comparison of General parameters. | |||
| Parameter | OD Tacrolimus (n = 24) |
Convention Tacrolimus (n = 24) | p value |
| Average age | 41.1 years | 43.7 years | 0.72 |
| Gender | 0.86 | ||
| Male | 19 (79.17%) | 20 (83.33%) | |
| Female | 5 (20.83%) | 4 (16.67%) | |
| Average weight | 57.9 kg | 61.5 kg | 0.67 |
| Type of donor | 0.59 | ||
| Related | 13 (54.17%) | 11 (45.83%) | |
| Un-related | 6 (25%) | 13 (54.17%) | |
| Cadaveric | 5 (20.83%) | 0 | |
| Time since treatment (mean) | 42.2 months | 46.6 months | 0.83 |
Basic Renal Disease causing ESRD has been recorded for each group in Table 2.
| Table 2: Basic Renal Disease Distribution for each Group. | ||
| OD Tacrolimus (n = 24) | Convention BD Tacrolimus (n = 24) | |
| Diabetic Nephropathy | 8 (33.33%) | 8(33.33%) |
| IgA Nephropathy | 5 (20.83%) | 4 (16.67%) |
| Nephrotic Syndrome | 4 (16.67%) | 1 (4.17%) |
| Chronic Interstitial Nephritis | 3 (12.5%) | 4 (16.67%) |
| Hypertensive Nephropathy | 2 (8.33%) | 4 (16.67%) |
| Chronic Glomerular Nephritis | 1 (4.17%) | 1 (4.17%) |
| Chronic Pyelonephritis | 1 (4.17%) | 1 (4.17%) |
| Congenital Disease | 0 | 1 (4.17%) |
Parameters at the time of the last follow-up were compared for each of the groups and are recorded in Table 3.
| Table 3: Parameters, at Last, Follow up. | |||
| Parameter | OD Tacrolimus | Conventional Tacrolimus | p value |
| Average weight (kg) | 64.6 | 66.6 | 0.78 |
| Average Tacrolimus dose (mg) | 2.7 mg | 2.15 | 0.82 |
| Average Dose mg/kg body weight | 0.04 | 0.03 | |
| Last Dose of tacrolimus | 3 | 2.6 | 0.69 |
| Average Sr. Creatinine (mg/dl) | 1.2 | 1.32 | 0.73 |
Average weight (64.6 kg vs. 66.6 kg), average tacrolimus dose (2.7 mg vs. 2.15 mg), average dose/kg body weight (0.04 mg/kg vs. 0.03 mg/kg), tacrolimus dose at last visit (3 mg vs. 2.6 mg), average Sr. Creatinine at last visit (1.2 mg/dl vs. 1.32 mg/dl), were comparable among both OD Tacrolimus and conventional BD Tacrolimus respectively which was statistically insignificant (p > .05).
New onset diabetes after transplant was recorded in 9 patients (37.5%) on conventional BD tacrolimus compared to 6 patients (25%) with once-daily tacrolimus. The difference was statistically significant (p = 0.041).
Post-transplant diabetes mellitus is defined as diabetes beyond 4 wks. Of transplant.
In this study very high incidence of post-transplant diabetes mellitus (PTDM), 5 patients (20.83%) were recorded in the Convention twice daily Tacrolimus group, compared to the Once Daily Tacrolimus group 1 patient (4.1%). The difference was statistically significant p = 0.17.
No previous study has shown such a huge benefit of reduction in the incidence of PTDM with the use of Once-a-day Tacrolimus, this needs to be addressed in larger randomized controlled trials in the future Table 4.
| Table 4: Incidence of post-transplant diabetes mellitus. | ||
| Duration to develop PTDM | OD Tacrolimus (n = 24) | Convention Tacrolimus (n = 24) |
| 1-week post-transplant | 3 (12.5%) | 2 (8.33%) |
| 2-weeks post-transplant | 0 | 1 (4.17%) |
| 3-weeks post-transplant | 2 (8.33%) | 0 |
| 1-month post-transplant | 0 | 1 (4.17%) |
| 2-months post-transplant | 1 (4.17%) | 0 |
| 1-year post-transplant | 0 | 3 (12.5%) |
| 2-years post-transplant | 0 | 1 (4.17%) |
| 3.8-years post-transplant | 0 | 1 (4.17%) |
We recorded a very low incidence of infections in the Once Daily Tacrolimus group. 1 patient (4.17%) as compared to 10 patients (41.67%) in the Conventional BD Tacrolimus group at 2 yrs. follow up. This difference was statistically very significant (p = 0.01).
Basiliximab Induction with 20 mg in two doses was statistically insignificant between the 2 groups. 11 patients in once daily tacrolimus with 5 being cadaveric transplants and 13 patients in the conventional BD tacrolimus group with no cadaveric transplants received induction Table 5.
| Table 5: Post Transplant Infections among two groups. | ||
| Type of Infection | OD group (n = 24) | Convention Tacrolimus group (n = 24) |
| UTI | 0 | 7 (29.17%) |
| Pneumonia | 0 | 1 (4.17%) |
| Mucor | 0 | 1 (4.17%) |
| TB Effusion | 0 | 1 (4.17%) |
| Military TB | 1 (4.17%) | 0 |
We have observed that there was 100% patient and graft survival at the end of two years in the OD Tac group, however.
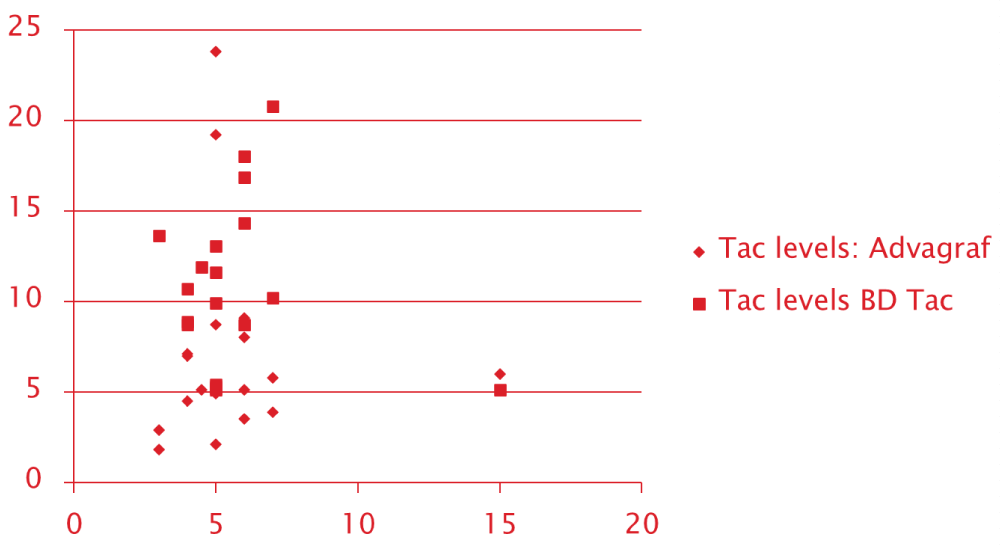
Scatter plot of the Initial 0-day Tacrolimus trough level for both groups recorded with 0.1 mg/kg/day of tacrolimus dose showed a more concentrated cluster around the acceptable range for OD tacrolimus group unlike with the conventional BD Tacrolimus. Scatter with conventional BD tacrolimus was much more diffuse and out-layered. This probably suggests the stable and sustained levels achieved with OD tacrolimus dosing Figure 1.
Figure 1: Scatter Plot of Tac trough levels for OD Tacrolimus and Conventional Tacrolimus.
Tacrolimus is a drug with a narrow therapeutic range and demonstrates inter‐and intra-patient pharmacokinetic (PK) variability [7]. Tacrolimus trough levels are monitored to guide dose adjustment, as it is highly correlated with tacrolimus AUC and subsequently clinical outcomes [4,8].
Conventional Tacrolimus is formulated for immediate release and is available for absorption till the proximal small bowel, while once daily Tacrolimus is a prolonged release formulation of Tacrolimus, available for absorption even at the distal small bowel and ascending colon. Since the expression of CYP3A4 and PgP reduce in the distal bowel, the pre-systemic metabolism is avoided and absorption continues [9].
There is a clear relationship between the complexity of the overall drug regimen and patient adherence. The more drugs and doses a patient had to remember, the greater the likelihood that some would be forgotten. Overall, they reported, “the best predictor of medication compliance seems to be simplicity. The simpler the prescription, the better the compliance [10].
Denhaerynck, et al. [11], found a weighted mean prevalence of nonadherence at 27.7% (range, 2% to 67%) in 10 studies that measured adherence by self-report. The prevalence of nonadherence in 2 studies that employed electronic event monitoring was 26% and 20%. Non-adherence was associated with poor clinical outcomes, contributing to a weighted mean of 19.9% of late AR episodes in 3 studies and 16.3% of graft losses in 8 studies. Distress over cosmetic and other side effects of immunosuppressive drugs also may trigger nonadherence, as noted by De Geest and Moons [12].
In order to reduce non-adherence, the concept of once-daily tacrolimus was introduced. It potentially improves inter- and intra-subject variability in exposure and improves compliance. OD tacrolimus therapeutically is equivalent to Twice daily Tacrolimus with the same therapeutic monitoring as noted in earlier studies [2-4].
Bakr MA, et al. [13] noted that renal function and rejection episodes showed no statistical significance among recipients of both groups. Despite slightly higher unit doses, there was no statistical difference regarding the tacrolimus trough levels, between the two groups.
Our single-center experience revealed that at almost similar doses, OD tacrolimus had a similar outcome to conventional tacrolimus on rejection episodes and graft survival at 2yrs post-transplant. The difference from previous studies was that the dose for OD tacrolimus was the same as conventional tacrolimus.
Helen F, et al. [14] in their study with OD Tac (N106) and standard-release Tac (N 95) recorded comparable eGFR at 12 months (58.8 ± 17 vs. 59.2 ± 18 mL/min, p = 0.307), New-onset diabetes (17 vs. 20%, p = 0.581), BK viremia (10 vs. 7%, p = 0.450), acute rejection (7vs. 16%, p = 0.067) or graft survival (97 vs. 95%, p = 0.301). In this study, OD Tac patients required fewer adjustments of doses suggesting stable levels. These findings were similar to the results recorded in our study.
Our study showed a lesser incidence of PTDM and a significantly lower incidence of post-transplant infections in OD tacrolimus groups compared to Conventional Tacrolimus which may be attributed probably to a steady state of levels with OD Tac. Unlike with Once daily tacrolimus conventional twice-daily Tac would provide 2 surges per day responsible for higher toxicity and complications. The highly significant difference in the incidence of PTDM & Infections has been recorded for the first time and probably needs to be evaluated in a large randomized controlled trial. This is recorded to be consistent and would translate to better post-transplant outcomes.
Limitations of our study are its retrospective design, non-randomized with the absence of pharmacokinetic characteristics, and small sample size.
OD Tacrolimus is comparable to conventional tacrolimus in its efficacy and safety. OD Tacrolimus however provides a more stable and steady state of blood levels which probably translates to a significantly lower incidence of PTDM and Post-transplant infections. This however needs to be corroborated in large RCT.
- Spencer CM, Goa KL, Gillis JC. Tacrolimus. An update of its pharmacology and clinical efficacy in the management of organ transplantation. Drugs. 1997 Dec;54(6):925-75. doi: 10.2165/00003495-199754060-00009. PMID: 9421697.
- Banas B, Krämer BK, Krüger B, Kamar N, Undre N. Long-Term Kidney Transplant Outcomes: Role of Prolonged-Release Tacrolimus. Transplant Proc. 2020 Jan-Feb;52(1):102-110. doi: 10.1016/j.transproceed.2019.11.003. Epub 2019 Dec 31. PMID: 31901329.
- Posadas Salas MA, Srinivas TR. Update on the clinical utility of once-daily tacrolimus in the management of transplantation. Drug Des Devel Ther. 2014 Sep 1;8:1183-94. doi: 10.2147/DDDT.S55458. PMID: 25210441; PMCID: PMC4155987.
- Mathew BS, Fleming DH, Jeyaseelan V, Chandy SJ, Annapandian VM, Subbanna PK, John GT. A limited sampling strategy for tacrolimus in renal transplant patients. Br J Clin Pharmacol. 2008 Oct;66(4):467-72. doi: 10.1111/j.1365-2125.2008.03251.x. Epub 2008 Jun 28. PMID: 18662286; PMCID: PMC2561121.
- Summary of Product Characteristics of OD 0.5 mg, 1 mg, 3 mg and 5 mg prolonged-release hard capsules; Astellas Pharma Ltd; June 2015. Available from: https://www.medicines.org.uk/emc/medicine/19814
- Sukkha S, Suansanae T, Iamrahong P, Wiwattanathum P. Trough Level and Tacrolimus Variability of Early Converted Once-Daily Tacrolimus: 1-Year Follow-up Study. Transplant Proc. 2020 Apr;52(3):775-779. doi: 10.1016/j.transproceed.2019.12.039. Epub 2020 Mar 4. PMID: 32143870.
- Musuamba FT, Mourad M, Haufroid V, Delattre IK, Verbeeck RK, Wallemacq P. Time of drug administration, CYP3A5 and ABCB1 genotypes, and analytical method influence tacrolimus pharmacokinetics: a population pharmacokinetic study. Ther Drug Monit. 2009 Dec;31(6):734-42. doi: 10.1097/FTD.0b013e3181bf8623. PMID: 19855314.
- Miura M, Satoh S, Niioka T, Kagaya H, Saito M, Hayakari M, Habuchi T, Suzuki T. Early phase limited sampling strategy characterizing tacrolimus and mycophenolic acid pharmacokinetics adapted to the maintenance phase of renal transplant patients. Ther Drug Monit. 2009 Aug;31(4):467-74. doi: 10.1097/FTD.0b013e3181ae44b9. PMID: 19571775.
- Miura M, Satoh S, Niioka T, Kagaya H, Saito M, Hayakari M, Habuchi T, Suzuki T. Early phase limited sampling strategy characterizing tacrolimus and mycophenolic acid pharmacokinetics adapted to the maintenance phase of renal transplant patients. Ther Drug Monit. 2009 Aug;31(4):467-74. doi: 10.1097/FTD.0b013e3181ae44b9. PMID: 19571775.
- Laederach-Hofmann K, Bunzel B. Noncompliance in organ transplant recipients: a literature review. Gen Hosp Psychiatry. 2000 Nov-Dec;22(6):412-24. doi: 10.1016/s0163-8343(00)00098-0. PMID: 11072057.
- Denhaerynck K, Dobbels F, Cleemput I, Desmyttere A, Schäfer-Keller P, Schaub S, De Geest S. Prevalence, consequences, and determinants of nonadherence in adult renal transplant patients: a literature review. Transpl Int. 2005 Oct;18(10):1121-33. doi: 10.1111/j.1432-2277.2005.00176.x. PMID: 16162098.
- De Geest S, Abraham I, Moons P, Vandeputte M, Van Cleemput J, Evers G, Daenen W, Vanhaecke J. Late acute rejection and subclinical noncompliance with cyclosporine therapy in heart transplant recipients. J Heart Lung Transplant. 1998 Sep;17(9):854-63. PMID: 9773856.
- Bakr MA, Nagib AM, Donia AF, Denewar AA, Abu-Elmagd MM, Abbas MH, Abdel-Rahman AM, Mashaly ME, Elsaftawy MM, Ghoneim MA. Comparative analysis for optimizing the modified release tacrolimus (Advagraf) after kidney transplantation: A prospective randomized trial. Saudi J Kidney Dis Transpl. 2018 Nov-Dec;29(6):1267-1273. doi: 10.4103/1319-2442.248303. PMID: 30588956.
- Fanous H, Zheng R, Campbell C, Huang M, Nash MM, Rapi L, Zaltzman JS, Prasad GV. A comparison of the extended-release and standard-release formulations of tacrolimus in de novo kidney transplant recipients: a 12-month outcome study. Clin Kidney J. 2013 Feb;6(1):45-49. doi: 10.1093/ckj/sfs169. Epub 2012 Jan 1. PMID: 23372940; PMCID: PMC3560378.