More Information
Submitted: July 18, 2024 | Approved: August 21, 2024 | Published: August 22, 2024
How to cite this article: Nadareishvili L, Mchedlishvili L, Nakaidze N, Dadiani M, Nizharadze D, Kutateladze M. Resolution of Chronic Bacterial Prostatitis with Bacteriophage-antibiotic Therapy. Int J Clin Virol. 2024; 8(2): 026-030. Available from: https://dx.doi.org/10.29328/journal.ijcv.1001059.
DOI: 10.29328/journal.ijcv.1001059
Copyright License: © 2024 Nadareishvili L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Resolution of Chronic Bacterial Prostatitis with Bacteriophage-antibiotic Therapy
Lia Nadareishvili1, Lasha Mchedlishvili1, Nata Nakaidze1*, Mariam Dadiani1, Dea Nizharadze1 and Mzia Kutateladze2
1Eliava Phage Therapy Center, Eliava Foundation, Tbilisi, Georgia
2Microbiology and Virology, G. Eliava Institute of Bacteriophages, Georgia
*Address for Correspondence: Nata Nakaidze, Eliava Phage Therapy Center, Eliava Foundation, Tbilisi, Georgia, Email: [email protected]
Chronic bacterial prostatitis is a commonly diagnosed genitourinary infection that presents significant challenges both in diagnosis and treatment. In an upcoming era of antibiotic resistance, and limited therapeutic options it becomes imperative to revise current guidelines and to provide more effective treatment strategies. At the Eliava Phage Therapy Center (Tbilisi, Georgia) we utilize bacteriophage therapy as an alternative approach against chronic bacterial infections. Bacteriophages, viruses that target and lyse specific bacterial cells, can be used as a stand-alone treatment or in conjunction with antibiotics. We present a case report of a patient with prostatitis caused by Escherichia coli infection, who prior to addressing our clinic, has been receiving antibiotic therapy without any positive effect. Our approach of combined use of antibiotics and phages was successful not only in complete clinical improvement but also in total bacterial eradication. This outcome shows the potential of bacteriophage therapy as a valuable adjunct to conventional antibacterials in the management of prostatitis.
Prostatitis, characterized by inflammation of the prostate gland, is a common condition with a lifetime prevalence rate of 1.8% to 8.2% [1]. According to the National Institutes of Health Classification, prostatitis is divided into the following categories: Acute bacterial prostatitis, Chronic Bacterial Prostatitis (CBP), Chronic non-bacterial prostatitis/Chronic pelvic pain syndrome, Inflammatory, Non-Inflammatory and Asymptomatic Inflammatory prostatitis [2]. Clinical manifestations mirror those of UTIs: increased urinary frequency, urgency, dysuria, discomfort in the pelvic area, abdominal discomfort, and low-grade fever [3]. Moreover, patients with CBP are usually diagnosed with recurrent urinary tract infections (UTIs) [4]. Interestingly, in some cases despite bacterial eradication, the symptoms still persevere [5]. This condition especially in the chronic form significantly alters the quality of life, affecting the mental and physical health of patients [6].
The main causative agents of CBP are Enterococcus faecalis and Escherichia coli [7]. It has been shown that E. coli caused acute prostatitis, and is associated with an increased biofilm formation [8], making it much more resistant to treatment. Other possible causative pathogens include gram-positive bacteria such as Staphylococcus and Streptococcus species, as well as atypical pathogens like Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma genitalium, Neisseria gonorrhoeae, etc; [9]. Depending on the causative agent the treatment approach varies significantly.
Antibiotic therapy stands as the primary treatment for CBP, complemented by alpha-blockers, anti-inflammatory medications, hormonal treatment, lifestyle adjustments, and in some cases, surgical interventions [10]. Nonetheless, the recurrent nature of CBP and escalating antibiotic resistance rates among patients highlight the pressing need to study the spectrum of pathogens and antibiotic resistance rates [11]. Bacteriophage therapy (BT), used in conjunction with antibiotics or as a stand-alone treatment presents a promising alternative for combating CBP, as evidenced by studies conducted by Letkiewicz, et al. [12], Johri, et al. [13], Stevens, et al. [14], Leitner, et al. [15], and Gorski, et al. [16].
Bacteriophages, or phages are viruses that are abundant in the natural environment. They have the remarkable ability to infect and lyse the host bacteria. Each phage, a bacterial virus, is specialized to target a specific bacteria. Upon encountering its target, a bacteriophage injects its genomic material, either DNA or RNA, into the bacterial cell, leading to its destruction. This group of viruses was introduced into clinical practice in the early 20th century, before the widespread use of antibiotic therapy.
Phage therapy has a long history of success in the treatment of infections in the Former Soviet Union Countries, where this group of viruses has been used as a mono-therapy, but also in some cases together with antibacterials. For today phage-antibiotic synergy is a promising approach to decrease the antibiotic resistance formation rate, biofilm penetration [17], and reduction of antibiotic dose intake [18].
At the Eliava Phage Therapy Center (EPTC) in Tbilisi, Georgia, we provide treatment to patients seeking BT for various chronic bacterial infections. The most common type of infection diagnosed at our clinic is the infection of the genitourinary system, including CBP. Drawing upon our clinic’s extensive experience with this pathology, we used a combined approach of phages and antibiotics in the treatment of this patient. We achieved complete amelioration of chief complaints and eradication of the bacterial infection.
In June 2023, a 65-year-old man sought treatment at the EPTC, where doctors confirmed the diagnosis of CBP. Upon presentation, his chief complaints included dysuria, increased urinary frequency, urinary urgency, and malodorous urine. Additionally, he reported experiencing high fever and extreme fatigue during acute infection episodes. Before his visit to EPTC, the patient has been on low-dose antibiotic therapy (Sulfamethoxazole and Trimethoprim) for five consecutive months, without experiencing any relief in his symptoms.
The patient was first diagnosed with CBP in April of 2022. He received emergency care in the US due to spiking fever, chills, body aches, numbness of fingers, and chest pain. The infection was attributed to Escherichia coli (>1.105CFU/mL), characterized by ESBL (Extended Spectrum Beta-Lactamase) and MDR (Multi-Drug-Resistant Organisms). The patient was administered IV Fosfomycin initially, but he experienced a severe adverse reaction to the medication. Consequently, he was switched to Bactrim (Sulfamethoxazole and Trimethoprim) for a duration of 6 weeks.
Before the hospitalization, the patient experienced a gradual decline in health over 8 weeks, during which the bacterial urine culture consistently showed negative results. Since then, he has experienced multiple episodes of acute bacterial infection, which has eventually become chronic in nature. The urine culture test remained positive for E. coli infection (Table 1). He received therapy with multiple antibiotics including Sulfamethoxazole and Trimethoprim, Fosfomycin, Penicillin, and Levofloxacin. The duration of time off of antibiotic therapy was gradually shortened, to the point the patient discontinued therapy; he noticed symptoms returning within 2-3 days.
| Table 1: Results of urine bacterial culture tests conducted in the US before the initiation of BT. | |
| Date | Urine Culture Test Results |
| 08.04.2022 | Escherichia coli >1.105 CFU/mL |
| 10.05.2022 | Escherichia coli >1.105 CFU/mL |
| 10.10.2022 | Negative: No growth |
| 22.12.2022 | Escherichia coli >1.105 CFU/mL |
| 17.02.2023 | Escherichia coli >1.105 CFU/mL |
The patient’s past medical history is significant for extensive antibiotic use and complicated surgeries, including perforated colon repair surgery in 2018, followed by two hernia repair surgeries and a shoulder replacement surgery. In July 2021, he was hospitalized due to abdominal obstruction. According to the patient, his genitourinary problems have slowly started, following this hospitalization. It is also worth noting that at 28 years old, he has undergone extensive IV antibiotic therapy, which was necessitated by a lacerated liver.
The urine culture tests conducted at the Eliava Analytic-Diagnostic Center in March of 2023, also confirmed an E. coli infection with a count of >1.104 CFU/mL. Additionally, a sperm culture showed the presence of E. coli>5.106 CFU/mL. The bacterial strain isolated from the urine revealed resistance to commercially available, phage preparations with fixed composition: Pyo phage, Intesti phage, Ses phage, and Enko phage. Therefore, the decision was made to develop a custom phage by the G. Eliava Institute of Bacteriophages, Microbiology, and Virology, using the patient’s specific bacterial strain.
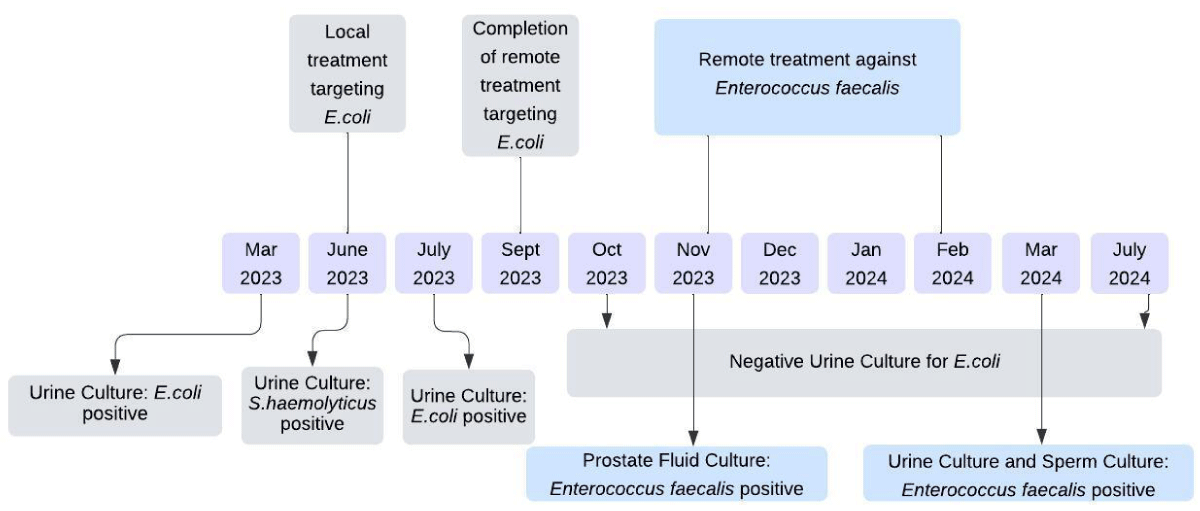
Local treatment was administered with customized phage at the EPTC from June 9 to June 23, 2023 (Figure 1). During this period, the urine culture test revealed Staphylococcus haemolyticus at a count of 3x103CFU/mL, which exhibited sensitivity to Pyo phage, intermediate sensitivity to Intesti, Fersis, Ses, and Enko phage. The sperm culture test remained negative at this time. Based on the sensitivity testing a decision was made to pursue the following combination therapy:
1. Pyo bacteriophage: 10 mL orally in the morning.
2. E. coli custom bacteriophage: 10 mL orally in the evening.
3. Ses bacteriophage: rectal suppository once a day.
4. Bactrim antibiotic: 160 mg twice a day. (The dosage and frequency remained unchanged since starting the phage treatment).
Figure 1: The timeline of treatment involves both ready-to-use phages, as well as custom-made phage preparations.
The therapy was given for 35 consecutive days followed by a ten-day break. The next two rounds of BT were administered over a period of 20 days, with again a ten-day break in between. After the first round of BT, a urine culture test was repeated in the US, which revealed a bacterial count up to 2.5 x 104 CFU/mL. Following the completion of BT, subsequent urine culture tests were performed seven times and returned negative results.
Throughout the treatment process, we also monitored the bacterial load in sperm ejaculate and prostate fluid. In November 2023, the prostate fluid culture revealed Enterococcus faecalis >5.105 CFU/mL. This month, without prior sensitivity testing, the decision was made to initiate Intesti phage therapy in combination with Levaquin. Intesti phage that includes active phage against Enterococcus was shipped to the US and the patient was provided with the following instructions: 10mL in the morning and 10 mL in the evening per os for 20 days, with a 15-day break between each curative period. This cycle was repeated three times. Tests performed in March at the Eliava diagnostic center revealed an E. faecalis sperm count of 5.103 and a urine culture test count of less than 1.103 CFU/mL.
The decrease in urinary bacterial load coincided with the improvement of the patient’s clinical complaints. By the middle of the last 20-day period of BT targeting an E. coli infection, there was a notable enhancement in the patient’s well-being. As of July 2024, he continues to remain symptom-free. No allergic reactions or adverse effects were documented throughout the phage therapy.
While prostate fluid analysis is valuable in CBP assessment, urine culture tests are more accessible for patients, and remain mandatory for the diagnosis [19]. Consequently, our evaluation of the patient’s health condition primarily relied on urine culture tests, supplemented by a couple of semen culture tests conducted in parallel. Even though the value of semen culture tests has been challenged in prostatitis, a study has also found a correlation between prostate and semen culture tests [20]. From our findings, we identified E. coli as the main cause of infection. The reduction in bacterial load aligned with the amelioration of the clinical symptoms. It is worth emphasizing that our analysis relied on lab tests performed locally at Eliava Diagnostic Center and patient-reported results from the US. Importantly, the findings from both laboratories were consistent with each other.
Since the patient was receiving a combination therapy of antibiotics and phages, it is hard to attribute improvements to one treatment over the other. However, it is clear that the use of Bactrim antibiotic alone did not yield clinical improvement for months, and only combination therapy - antibiotic with bacteriophage proved effective in achieving positive results. This shows the potential of phage and antibiotic synergy, which has been described by multiple authors [21,22]. In vitro studies have demonstrated positive synergetic effects against MDR uropathogenic E. coli [23].
The formation of bacterial biofilms presents a significant challenge to the effectiveness of antibiotics, particularly in cases where E. coli is a well-known contributor to biofilm production [24]. Although antibiotics are typically the primary treatment for CBP [9], their efficacy can be limited, especially in cases of refractory infections, necessitating a switch to different antibiotic classes. Biofilm formation by various etiological agents such as Staphylococcus spp, Enterococcus spp, E. coli, Enterobacteriaceae, and Pseudomonas aeruginosa is common [25]. Considering this challenge, the combined use of phage therapy and antibiotics could offer a viable option for the treatment of CBP, as phages and phage-encoded enzymes have been shown as potential agents for biofilm penetration [26]. Moreover, the combination use of phages and antibiotics is promising against not only biofilm penetration but also for decreasing the risk of phage resistance development [27]. Our case report is another example, that showcases the importance of using phages in cases where there is severe resistance against antibiotics alone, possibly due to biofilm formation.
The human bladder was once considered a sterile environment, but this myth has since been debunked by various studies [28,29]. It has been shown that the urinary microbiome plays a key role in the maintenance of overall urinary health and differs in “healthy” patients vs. those with genitourinary infections [30]. Phages, along with other microorganisms such as bacteria, fungi, and protozoa form the human bladder microbiome [31]. Thus, the urinary microbiome is sustained by a delicate balance between pathogenic and non-pathogenic bacteria. Disruptions to this balance can occur when the composition of organisms changes. During a bacterial infection, specific phages target and kill the infectious agent - host bacteria while simultaneously helping to restore the microbial equilibrium. In nature, phages and bacteria engage in continuous co-evolutionary processes. Introducing exogenous phage preparations into the urinary microbiome can positively impact this balance by reducing the number of pathogenic bacteria and thereby promoting the proliferation of beneficial bacteria.
Phage therapy has been widely utilized in clinical practice in Georgia for decades. At Eliava Phage Therapy Center, we employ phage preparations to combat infectious diseases affecting various organ systems. In recent years there has been a marked interest in BT, as evidenced by a growing number of registered clinical trials and an expanding number of institutions practicing phage therapy. Based on the results of double-blind studies, phage preparations could potentially have broader applications in the future, challenging the current status quo of antibiotics.
The treatment of infectious diseases using various antibacterial solutions, including phages, is approximately comparable from a financial perspective. However, phage therapy necessitates experienced scientists for the selection and development of specific, high-quality phage preparations, which can be a costly process and, for now, is only conducted by a couple of Institutions, such as the Eliava Institute. If the isolated bacterial strain is sensitive to an already developed and commercially available phage preparation, the costs associated with phage therapy can be nearly equivalent to conventional treatment of infectious complications.
Emerging antibiotic resistance poses a significant challenge in the treatment of chronic bacterial infections. We used a combination of antibiotics and bacteriophage preparations for treating chronic bacterial prostatitis and achieved both complete clinical improvement and eradication of E. coli-caused infection. We believe this case report once again demonstrates the effectiveness of synergetic treatment against chronic urogenital infections.
The authors would like to express their gratitude to the patient who generously consented to participate in this case report and was allowed to share the clinical information.
- Holt JD, Garrett WA, McCurry TK, Teichman JM. Common questions about chronic prostatitis. Am Fam Physician. 2016; 93(4):290-296. Available from: https://pubmed.ncbi.nlm.nih.gov/26926816/
- Vaidyanathan R, Mishra VC. Chronic prostatitis: Current concepts. Indian J Urol. 2008; 24(1):22-27. Available from: https://doi.org/10.4103%2F0970-1591.38598
- Meyrier A, Fekete T. Chronic bacterial prostatitis. UpToDate. 2023.
- Khan FU, Ihsan AU, Khan HU, Jana R, Wazir J, Khongorzul P, et al. Comprehensive overview of prostatitis. Biomed Pharmacother. 2017; 94:1064-1076. Available from: https://doi.org/10.1016/j.biopha.2017.08.016
- Stamatiou KN, Moschouris H. A prospective interventional study in chronic prostatitis with emphasis to clinical features. Urol J. 2014; 11(4):1829-1833. Available from: https://pubmed.ncbi.nlm.nih.gov/25194085/
- McNaughton Collins M, Pontari MA, O'Leary MP, Calhoun EA, Santanna J, Landis JR, et al. Chronic Prostatitis Collaborative Research Network. Quality of life is impaired in men with chronic prostatitis: The Chronic Prostatitis Collaborative Research Network. J Gen Intern Med. 2001; 16(10):656-662. Available from: https://doi.org/10.1111/j.1525-1497.2001.01223.x
- Heras-Cañas V, Gutiérrez-Soto B, Serrano-García ML, Vázquez-Alonso F, Navarro-Marí JM, Gutiérrez-Fernández J. Prostatitis crónica bacteriana. Estudio clínico y microbiológico de 332 casos. Med Clin (Barc). 2016; 147(4):144-147. Available from: https://doi.org/10.1016/j.medcli.2016.05.018
- Kanamaru S, Kurazono H, Terai A, Monden K, Kumon H, Mizunoe Y, et al. Increased biofilm formation in Escherichia coli isolated from acute prostatitis. Int J Antimicrob Agents. 2006; 28 Suppl 1. Available from: https://doi.org/10.1016/j.ijantimicag.2006.05.006
- Lipsky BA, Byren I, Hoey CT. Treatment of bacterial prostatitis. Clin Infect Dis. 2010; 50(12):1641-1652. Available from: https://doi.org/10.1086/652861
- Duclos AJ, Lee CT, Shoskes DA. Current treatment options in the management of chronic prostatitis. Ther Clin Risk Manag. 2007; 3(4):507-512. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2374945/
- Trinchieri A, Abdelrahman KM, Bhatti KH, Bello JO, Das K, Gatsev O, et al. Spectrum of causative pathogens and resistance rates to antibacterial agents in bacterial prostatitis. Diagnostics (Basel). 2021; 11(8):1333. Available from: https://www.mdpi.com/2075-4418/11/8/1333#
- Letkiewicz S, Międzybrodzki R, Kłak M, Jończyk E, Weber-Dąbrowska B, Górski A. The perspectives of the application of phage therapy in chronic bacterial prostatitis. FEMS Immunol Med Microbiol. 2010; 60(2):99-112. Available from: https://doi.org/10.1111/j.1574-695x.2010.00723.x
- Johri AV, Johri P, Hoyle N, Nadareishvili L, Pipia L, Nizharadze D. Case report: Successful treatment of recurrent E. coli infection with bacteriophage therapy for patient suffering from chronic bacterial prostatitis. Front Pharmacol. 2023; 14:1243824. Available from: https://doi.org/10.3389%2Ffphar.2023.1243824
- Stevens RH, Zhang H, Kajsik M, Płoski R, Rydzanicz M, Sabaka P, Šutovský S. Successful use of a phage endolysin for treatment of chronic pelvic pain syndrome/chronic bacterial prostatitis. Front Med (Lausanne). 2023; 10:1238147. Available from: https://doi.org/10.3389%2Ffmed.2023.1238147
- Leitner L, Sybesma W, Chanishvili N, Goderdzishvili M, Chkhotua A, Ujmajuridze A, et al. Bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: A randomized, placebo-controlled, double-blind clinical trial. BMC Urol. 2017; 17(1):90. Available from: https://doi.org/10.1016/s1473-3099(20)30330-3
- Górski A, Jończyk-Matysiak E, Łusiak-Szelachowska M, Międzybrodzki R, Weber-Dąbrowska B, Borysowski J, et al. Phage therapy in prostatitis: Recent prospects. Front Microbiol. 2018; 9:1434. Available from: https://doi.org/10.3389%2Ffmicb.2018.01434
- Ferriol-González C, Domingo-Calap P. Phages for biofilm removal. Antibiotics (Basel). 2020; 9(5):268. Available from: https://doi.org/10.3390/antibiotics9050268
- Li X, He Y, Wang Z, Wei J, Hu T, Si J, et al. A combination therapy of phages and antibiotics: Two is better than one. Int J Biol Sci. 2021; 17(13):3573-3582. Available from: https://doi.org/10.7150%2Fijbs.60551
- Nickel JC. Classification and diagnosis of prostatitis: A gold standard? Andrologia. 2003; 35(3):160-167. Available from: https://doi.org/10.1046/j.1439-0272.2003.00557.x
- Mobley DF. Semen cultures in the diagnosis of bacterial prostatitis. J Urol. 1975; 114(1):83-85. Available from: https://doi.org/10.1016/s0022-5347(17)66949-0
- Valério N, Oliveira C, Jesus V, Branco T, Pereira C, Moreirinha C, et al. Effects of single and combined use of bacteriophages and antibiotics to inactivate Escherichia coli. Virus Res. 2017; 240:8-17. Available from: https://doi.org/10.1016/j.virusres.2017.07.015
- Łusiak-Szelachowska M, Międzybrodzki R, Drulis-Kawa Z, Cater K, Knežević P, Winogradow C, et al. Bacteriophages and antibiotic interactions in clinical practice: What we have learned so far. J Biomed Sci. 2022; 29(1):23. Available from: https://doi.org/10.1186/s12929-022-00806-1
- Malik S, Nehra K, Rana JS. Bacteriophage cocktail and phage-antibiotic synergism as promising alternatives to conventional antibiotics for the control of multi-drug-resistant uropathogenic Escherichia coli. Virus Res. 2021; 302:198496. Available from: https://doi.org/10.1016/j.virusres.2021.198496
- Soto SM, Smithson A, Martinez JA, Horcajada JP, Mensa J, Vila J. Biofilm formation in uropathogenic Escherichia coli strains: Relationship with prostatitis, urovirulence factors, and antimicrobial resistance. J Urol. 2007; 177(1):365-368. Available from: https://doi.org/10.1016/j.juro.2006.08.081
- Mazzoli S. Biofilms in chronic bacterial prostatitis (NIH-II) and in prostatic calcifications. FEMS Immunol Med Microbiol. 2010; 59(3):337-344. Available from: https://doi.org/10.1111/j.1574-695x.2010.00659.x
- Azeredo J, García P, Drulis-Kawa Z. Targeting biofilms using phages and their enzymes. Curr Opin Biotechnol. 2021; 68:251-261. Available from: https://doi.org/10.1016/j.copbio.2021.02.002
- Tagliaferri TL, Jansen M, Horz HP. Fighting pathogenic bacteria on two fronts: Phages and antibiotics as a combined strategy. Front Cell Infect Microbiol. 2019; 9:22. Available from: https://doi.org/10.3389%2Ffcimb.2019.00022
- Ackerman AL, Chai TC. The bladder is not sterile: An update on the urinary microbiome. Curr Bladder Dysfunct Rep. 2019;14(4):331-341. Available from: https://doi.org/10.1007/s11884-019-00543-6
- Hilt EE, McKinley K, Pearce MM, Rosenfeld AB, Zilliox MJ, Mueller ER, et al. Urine is not sterile: Use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014; 52(3):871-876. Available from: https://doi.org/10.1128%2FJCM.02876-13
- Schneeweiss J, Koch M, Umek W. The human urinary microbiome and how it relates to urogynecology. Int Urogynecol J. 2016; 27(9):1307-1312. Available from: https://doi.org/10.1007/s00192-016-2944-5
- Miller-Ensminger T, Garretto A, Brenner J, Thomas-White K, Zambom A, Wolfe AJ, et al. Bacteriophages of the urinary microbiome. J Bacteriol. 2018; 200(7). Available from: https://doi.org/10.1128%2FJB.00738-17